Theoretical calculation of equilibrium constant
A molecule may exist in any of series of energy level. It is the distribution of energy level that determines the equilibrium state of the system. for real molecules The energy level pattern would be much complicated .
We need to know two things in order to calculate the numeric value of the equilibrium constant:
· the balanced equation for the reaction system, including the physical states of each species.
· the equilibrium concentrations or pressures of each species that occurs in the equilibrium expression, or enough information to determine them. These values are substitued into the equilibrium expression and the value of the equilibrium constant is then calculated
If we know the standard state free energy change, Go, for a chemical process at some temperature T, we can calculate the equilibrium constant for the process at that temperature using the relationship between Go and K.
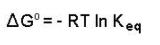
Rearrangement gives
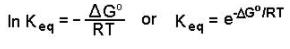
· into the equilibrium expression and solve for K
benyamin jafaryan
|